Iflaviridae





The Iflaviridae share the same characteristics with the Dicistroviridae in terms of structure and the dimension of the virion, but they can be easily distinguished by their genome organization. These viruses also have a linear ssRNA(+) genome, ranging from 8.8 to 9.7 kb, a Vpg protein covalently bounded to the 5’ terminus and a polyA tail at the 3’ terminus. They also have a long UTR at the 5’ end containing an internal ribosome entry site (IRES) and a 3' UTR. However, Iflaviruses have only one ORF that encodes both structural (capsid) and non-structural proteins (Chen and Siede, 2007).
Sacbrood virus (SBV)
SBV is the first fully sequenced virus of the honey bee (Ghosh et al., 1999). It is the most widely distributed of all honey bee viruses and the only virus that is associated with overt disease in the hive (Chen and Siede, 2007), In fact SBV has been reported from every continent (Ellis and Munn, 2005). It attacks both brood and adult stages of bees but causes no obvious symptoms in adults. Transmission within a colony occurs via nurse bees that become infected whilst removing larvae killed by SBV. Nurse bees, feeding the larvae and exchanging food with other adult bees, can spread the virus
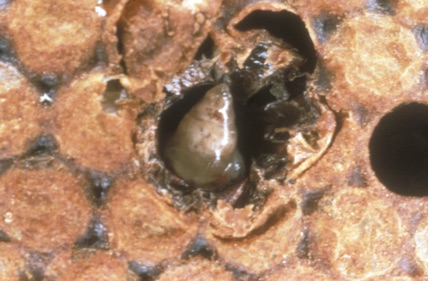
Fig 1: Sacbrood virus affected larva. Typical water-filled sac-like larva; this is the pathognomonic symptom of Sacbrood disease. (Crown copyright, National Bee Unit, FERA).
throughout the colony. Infected foraging bees spread the virus via their glandular secretions as they collect pollen. Young larvae become infected with the virus by ingesting virus-contaminated food (Chen and Siede, 2007).
Infected larvae fail to pupate properly, in particular they fail to shed their final layer of skin and a large amount of fluid (containing millions of SBV particles) accumulates between the body and this unshed skin. Affected larvae appear to be a water-filled sac, giving the disease its name. After death the larvae dry out and take on a brown scale like appearance. Infection with SBV can be readily diagnosed in the field because of these characteristic symptoms produced in the diseased brood. (Figure 1; Chen and Siede, 2007).
The frequency of SBV infection in spring and summer may be significantly higher than in autumn (Tentcheva et al., 2004b). In fact during spring and summer, rich sources of pollen and nectar stimulate brood rearing and a great number of new workers hatch
rom the brood cells; thus increasing the possibility that SBV may attack the developing honey bees and multiply in the colonies.
SBV infection has been associated with Varroa mite infestation as SBV has been detected in large quantities in adult bees from Varroa mite–infested colonies, but Varroa has not been shown to act as a vector in transmitting SBV (Berenyi et al., 2006).
Deformed wing virus (DWV), Varroa destructor virus (VDV-1) and Kakugo virus (KV)
DWV, VDV-1 and KV are three closely related iflaviruses of the honey bee. In fact DWV and VDV-1 have a genetic similarity of 84% and an amino acid similarity of 97% when sequences of the VP1 part of the genome are aligned (Ongus, 2006). Similarly, DWV and KV share 97% of the genome and a polyprotein similarity of 98% (Fujiyuki et al., 2004; Ongus, 2006), however despite the genetic similarity, these viruses have been reported ot produce different symptoms. DWV infections lead to anatomic deformity (deformed wings) (Bailey and Ball, 1981) and KV infections results in changes in behaviour (aggressive

Fig 1.11: DWV symptoms (adult bee). The honey bee appears less coloured than normal and the wings appear shrunken, atrophic and deformed. (Crown copyright, National Bee Unit, FERA).
bees) (Fujiyuki et al., 2004). No clear symptomatology has been described for VDV-1, as it is frequently mixed with DWV even in the same bee; so the symptoms of VDV-1 could be covered by the symptoms of DWV or simply VDV-1 could be a sub-clinical disease of the honey bee.
The most important and well known between these three viruses is DWV. It was isolated from adult bees in Japan (Bailey and Ball, 1991). The infection by DWV has so far been reported in Europe, North America, South America, Africa, Asia, and the Middle East (Allen and Ball, 1996; Antunez et al., 2006; Ellis and Munn, 2005). It has never been reported in Oceania until now.
DWV can cause well known symptoms in infected bees, although it does not usually trigger colony collapse alone. Typical disease symptoms of DWV infection are shrunken, deformed wings (hence, the name of the disease) (Figure 1.11), decreased body size, and discolouration in adult bees. However, the mechanism by which DWV causes the morphological deformities of the infected hosts is unclear (Chen and Siede, 2007).
All the prevalence surveys have shown that DWV is the most prevalent infection in A. mellifera. Research conducted from Yan Ping Chen in the United States showed that DWV infection occurred in all (100%) of the apiaries investigated (Chen and Siede, 2007). Tentcheva obtained similar results and DWV was detected in over 97% of French apiaries when the adult bee populations were examined (Tentcheva et al., 2004b). Some seasonal variation in virus incidence was observed and the frequency of DWV infection in both adult bees and pupae increased considerably from summer to autumn during the year (Tentcheva et al., 2004b). Other researchers, seems to agreed that DWV is the most prevalent virus of the honey bee ( Berenyi et al., 2006; Nielsen et al., 2008; Baker and Shoroeder, 2008; Forgach et al., 2008; Teixeira et al., 2008).
Laboratory and field studies showed that the Varroa mite is an active vector of the DWV (the virus can actually reply into the mite, so Varroa is not just a mechanical vector) (Martin et al., 1998; Bowen-Walker et al., 1999; Ongus, 2006). Varroa mites appeared to be DWV positive in 100% of French apiaries (Tentcheva et al., 2004b). Despite this evidence it was impossible to find the site of DWV replication in the Varroa mite by immunolocalization (Santillan-Galicia et al., 2008).
KV was described by Fujiyuki as a virus capable of increasing aggressive behaviour in the honey bee (Fujiyuki et al., 2004). Fujiyuki presented a giant hornet (Vespa mandarinia japonica) to the guard bees. He then analysed the attacking and the non-attacking bees and he found that in the brain of the aggressive bees was a virus that he named Kakugo which means “ready to attack” in Japanese (Fujiyuki et al., 2004). Because of the close relationship between KV and DWV (almost genetically indistinguishable) needs to be clarified whether they represent two different variants of the same virus, or and indeed whether DWV may be able to cause similar change in behaviour as KV.
Ongus demonstrated that VDV-1 and DWV were present only in Varroa parasitised honey bees (Ongus, 2006 ). This is confirmed by the fact that in areas where Varroa is never been reported (e.g. Kenya and north Sweden), VDV and DWV are also absent (Oldroyd, 1999; Yue and Genersch, 2005). This suggests that VDV-1 and DWV are both strongly associated with the Varroa mite, and that they are Varroa viruses adapted to replicate in honey bees.
Slow bee paralysis virus (SBPV)
SBPV is the most recent honey bee virus to be sequenced (de Miranda et al., 2010b). It remains unassigned, but the genomic characteristics suggest it is likely to belong to the Iflaviruses. This virus was described by Bailey while he was isolating CBPV and ABPV (Bailey and Woods, 1974). What he found was a virus with slightly different characteristics (30 nm in diameter, sedimentation 176S and buoyant density of 1.35 g/ml) that didn't react with any antibody against CBPV and ABPV and had the ability to kill injected bees in about 12 days. In addition the symptoms were different to those of ABPV and CBPV infection; in this case honey bees showed by paralysis of the anterior legs for a couple of days before death. It was named slow bee paralysis virus to differentiate it from the relatively quick disease progression that is it possible to see in CBPV and ABPV infection. Bailey theorised that this virus could be responsible to some the many unexplained colony losses that affect honey bee breeding every year (Bailey and Woods, 1974).
SBPV seems to be extremely rare, having been identified positively in the laboratory only in Britain, Fiji and Western Samoa (Allen and Ball, 1996; Martin et al., 1998; Anderson, 1990) despite being included in many of the surveys (Hornitzky, 1987; Ball and Allen, 1988; Topolska et al., 1995; Nordström et al., 1999; Todd et al., 2007)
As it was never reported in field studies, many scientists were keen to think that it was just a laboratory variant of a previously described bee virus (de Miranda, personal communication). This hypothesis can no longer be sustained as recent findings in the field of the sequences of SBPV clearly demonstrate that this is a virus, present in the environment with a pathogenetic role for the honey bees that needs to be clarified in the near future (de Miranda et al., 2010b).



Citation: Cordoni, G.; 2011. Epidemiology and taxonomy of honey bee viruses in England and Wales. PhD thesis. University of Surrey.
Developed by
Dr. Guido Cordoni DVM, PGDip, PhD, MRCVS